Confidently rule out melanoma with the DermTech® Melanoma Test1
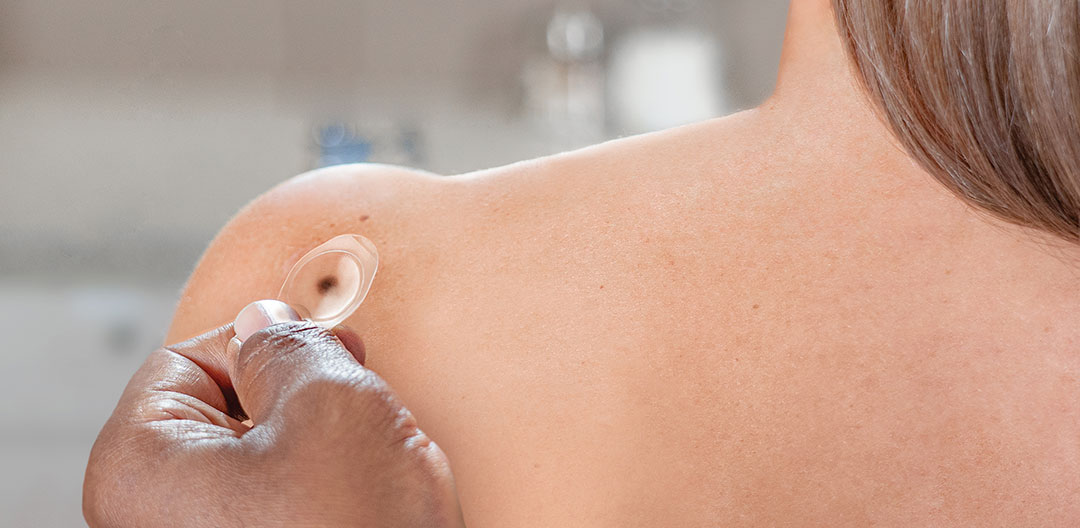
Get objective, actionable and easy to interpret results to inform the clinical management of pigmented lesions.1,3

No biomarkers detected
The tested lesion is unlikely to be melanoma.
Negative predictive value (NPV) 99%.1,5
NPV indicates the probability the lesion is not a melanoma.
Biomarker(s) detected
The tested lesion has a higher probability of being melanoma.
BIOMARKER(S) | APPROXIMATE PPV |
LINC00518 Only6 | 15% |
PRAME Only6 | 19% |
LINC00518 & PRAME1 | 67% |
BIOMARKER(S) | APPROXIMATE PPV |
LINC00518 Only6 | 15% |
PRAME Only6 | 19% |
LINC00518 & PRAME1 | 67% |
Positive predictive value (PPV) indicates the probability the lesion is a melanoma.
“The DermTech Melanoma Test provides objective genomic information that we can’t obtain any other way. The high 99% NPV helps clinicians confidently differentiate benign lesions from potentially malignant lesions.”
— Laura Ferris, MD, PhD
Professor and Chair of the UNC Department of Dermatology
Recommended use of the DermTech Melanoma Test

The DermTech Melanoma Test is indicated for use on pigmented lesions that:
- Meet one or more ABCDE criteria
- Have a diameter of ≥ 5mm
- Are on patients 18 years or older
The DermTech Melanoma Test reliably rules out melanoma with a 99% NPV and provides objective, actionable results to guide the management of clinically atypical pigmented lesions.
May be appropriate for patients who:7
- Have pigmented lesions with a low-to-moderate concern for malignancy
- Have difficult-to-biopsy lesions
- Have lesions in cosmetically sensitive areas
- Are needle-averse and seeking a non-invasive option
- Are poor biopsy candidates
Not appropriate for lesions:
- On palms of hands, soles of feet, nails, mucous membranes or hair covered areas that cannot be trimmed
- That are ulcerated or bleeding
How to use the DermTech Melanoma Test
Learn how to use the DermTech Smart Stickers to collect skin cells from an equivocal pigmented lesion. It takes less than 5 minutes per lesion and can be performed by medical staff or at home under supervision.

Easy to order. Easy to collect. Easy to read.

ORDER
Adhesive Skin Collection Kits included in the price of the test.b To order, contact us or ask your representative.

COLLECT

RECEIVE
Results are returned from the DermTech Gene Lab to your office typically within 5 business days.
NEXT STEPS
Clear, actionable results for atypical pigmented lesions. Negative: 99% probability the lesion is not melanoma.1,2,5 Positive: Higher probability of being melanoma.
Download Test Requisition Form
Adopt DermTech into your practice today.
aNCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
bDistribution of the Adhesive Skin Collection Kit is included in the reimbursement to DermTech for conducting the DermTech Melanoma Test.
cPlease refer to LCD #38151 for additional Medicare coverage criteria.
References:
1. Gerami P, et al. J Am Acad Dermatol. 2017;76(1):114-120. 2. Jackson S, et al. SKIN J Cutan Med. 2020;4(2):105-110. 3. Ferris L, et al. Dermatol Online J. 2019;25(5):2. 4. Yao Z, et al. J Drugs Dermatol. 2017;16(10):979-986. 5. Skelsey M, et al. SKIN J Cutan Med. 2021;5(5):512-523. 6. Data on file. DermTech, Inc. 7. Brouha B, et al. J Drugs Dermatol. 2020;19(3):257-262. 8. Brouha B, et al. SKIN J Cutan Med. 2021;5(1):13-18. 9. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Melanoma: Cutaneous V.2.2021. © National Comprehensive Cancer Network, Inc. 2021. All rights reserved. Accessed October 31, 2021. To view the most recent and complete version of the guideline, go online to NCCN.org.

