Meet the DermTech® Melanoma Test
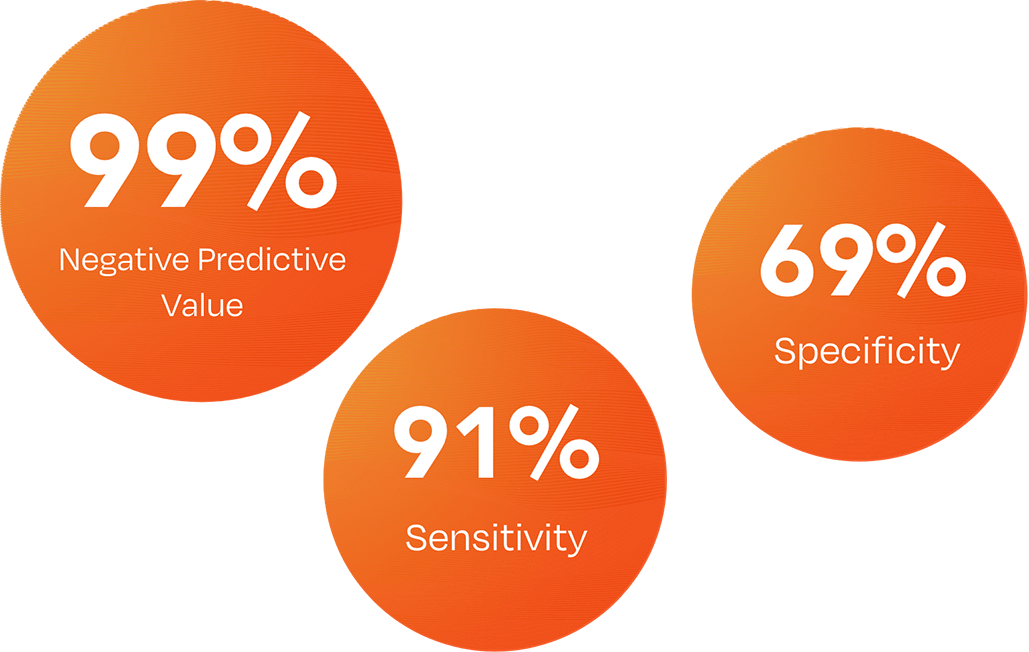
Specificity is the proportion of people without disease who test negative.
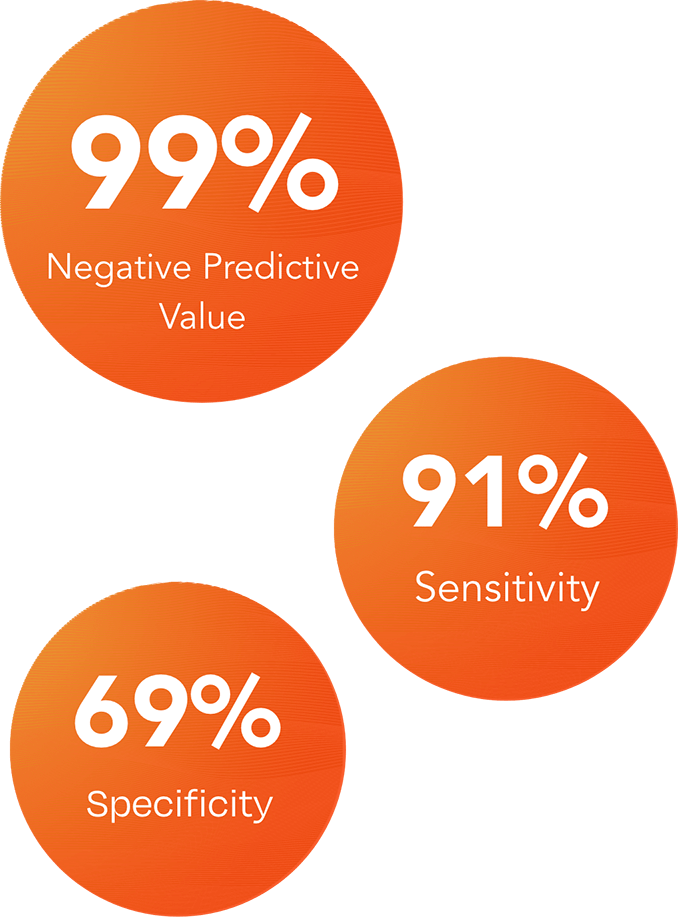
The DermTech Melanoma Test has a 99% negative predictive value (NPV), meaning that with a negative test result, there is a 99% probability that the lesion is not melanoma.
DermTech Melanoma Test2

Many early-stage melanomas resemble benign nevi.3,4
Clinical visual assessment is by nature subjective and presents challenges in the management of equivocal pigmented lesions. What is seen as clearly benign or clearly melanoma may vary by clinician.
The DermTech Melanoma Test reliably rules out melanoma with a 99% NPV and provides objective, actionable, easy to interpret results to guide the management of clinically equivocal pigmented lesions.
Harnessing Precision Genomics in Dermatology
Certain genomic abnormalities precede morphologic changes.4,5,6
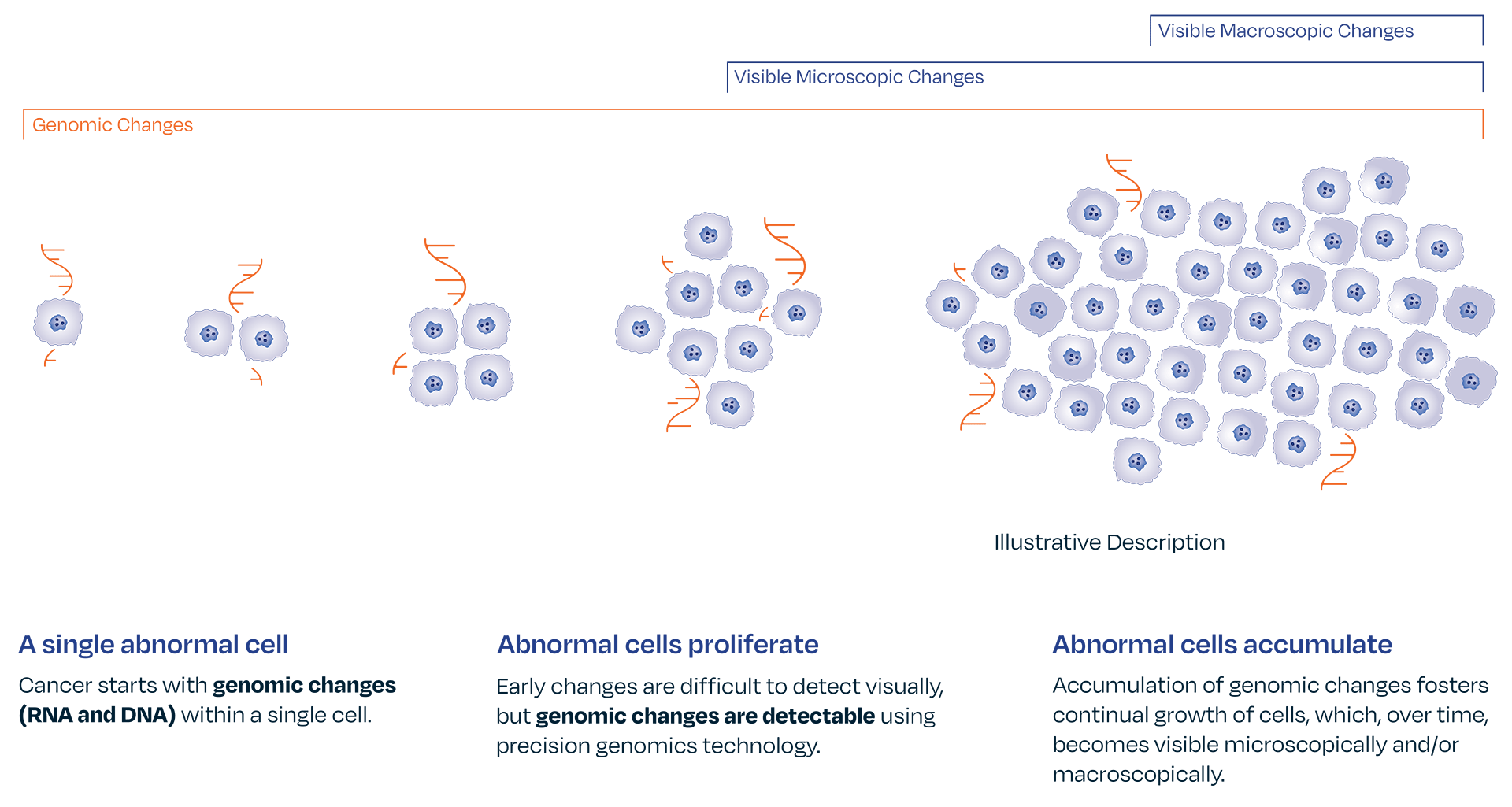
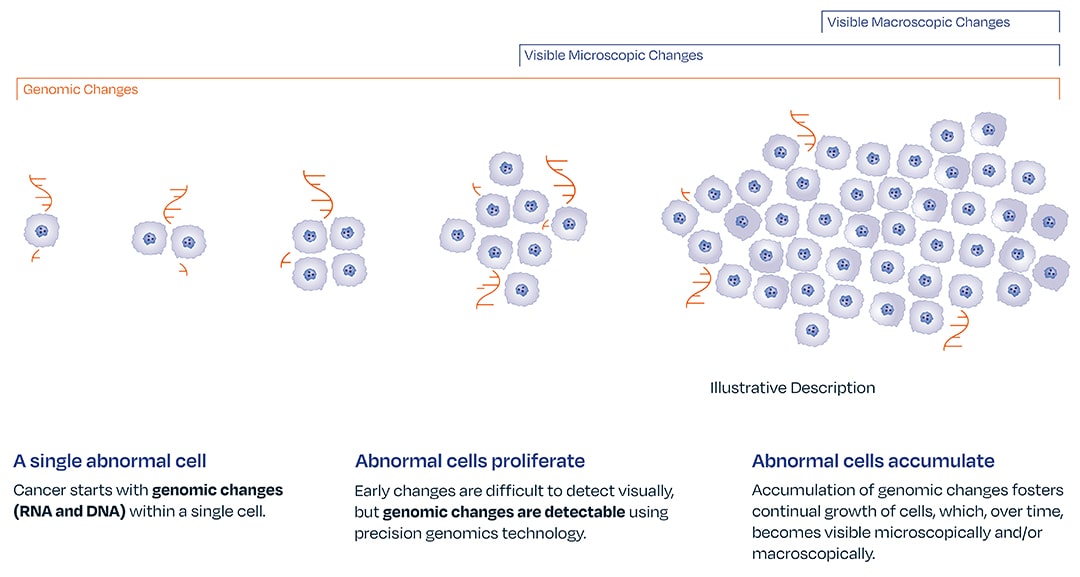
Genomic changes drive disease progression. The absence of gene expression by optimized melanoma associated genomic markers LINC00518 and PRAME can give a provider confidence in ruling out melanoma. The DermTech Melanoma Test’s has a 99% negative predictive value, meaning there is a 99% probability that a negative lesion is not melanoma.
The innovative DermTech Gene Lab is CLIA certified, JCO accredited, and licensed by the New York State Department of Health.
Adopt DermTech into your practice today.
References:
1. Gerami P, et al. J Am Acad Dermatol. 2017;76(1):114-120. 2. Swetter SM, et al. J Am Acad Dermatol. 2019;80(1):208-250. 3. SilvaJH, et al. Clinics (Sao Paulo). 2011;66(3):493-499. 4. Malvehy J, et al. Br J Dermatol. 2014;171(5):1099-1107. 5. Skelsey M, et al. SKIN J Cutan Med. 2021;5(5):512-523. 6. Shain AH, et al. Cancer Cell. 2018;34(1):45-55.e4.



